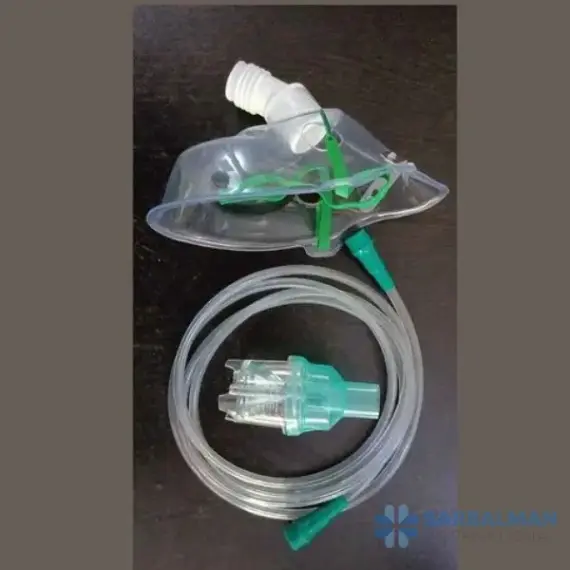
Nebulizer Face Mask Kit
Free!
Description
A Nebulizer Face Mask Kit is a ready-to-use set for delivering inhaled medications as a fine mist to the lungs. It typically includes a soft, anatomically shaped mask, a medication chamber (nebulizer cup), oxygen/nebulizer tubing, and an adjustable head strap. The mask allows therapy for patients who cannot use a mouthpiece, making treatments easier for children, older adults, and anyone short of breath during an attack. Clear materials and smooth edges support comfort and visual monitoring.
Key features and benefits:
• Complete kit: mask, nebulizer cup, tubing, and strap for immediate setup
• Soft, contoured mask with venting and a gentle seal to improve comfort and reduce leaks
• Transparent components for easy observation of breathing, condensation, and medication level
• Consistent aerosol delivery when used with standard pneumatic nebulizer compressors
• Standard connections for compatibility with common tubing and equipment
• Adult and pediatric sizes; color cues and clear size markings for fast selection
• Single-use sterile options to reduce cross-contamination; reusable options available per facility policy
• Latex-free, skin-friendly materials; phthalate-reduced options for sensitive users
Typical applications:
• Home and clinic treatments for asthma, COPD, bronchiolitis, and post-operative respiratory care
• Emergency departments, wards, and EMS during acute exacerbations
• Pediatrics, geriatrics, and patients with limited hand strength or coordination
Comparison and selection notes:
• Versus a mouthpiece: a mask is easier for anxious, fatigued, or pediatric patients; a mouthpiece may reduce facial drug deposition for cooperative adults
• Versus metered-dose inhalers with spacers: MDIs are highly portable; nebulizers provide continuous delivery with less technique dependence during distress
Quality and compliance considerations:
• Choose products made under a recognized medical device quality system (e.g., ISO 13485)
• Prefer patient-contact materials evaluated for biocompatibility (e.g., ISO 10993)
• Confirm clear use instructions and validated sterilization or reprocessing guidance