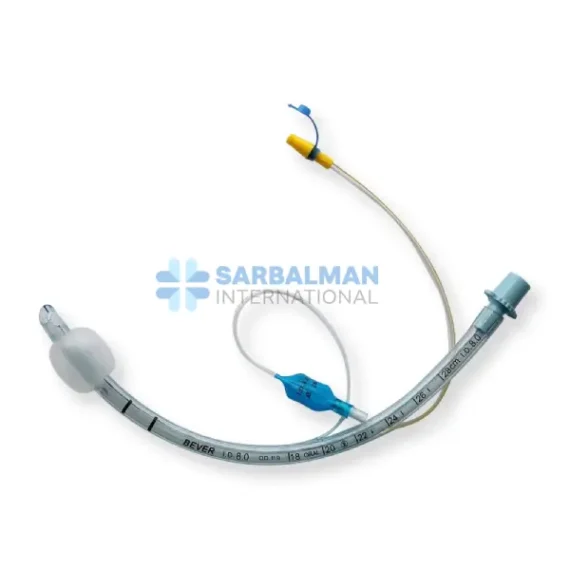
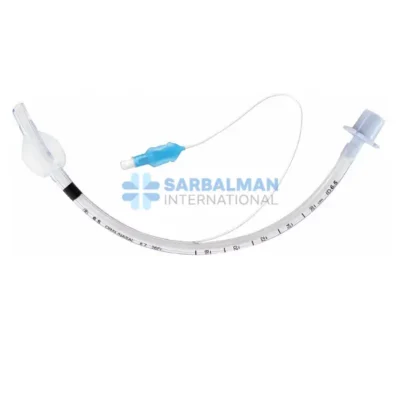
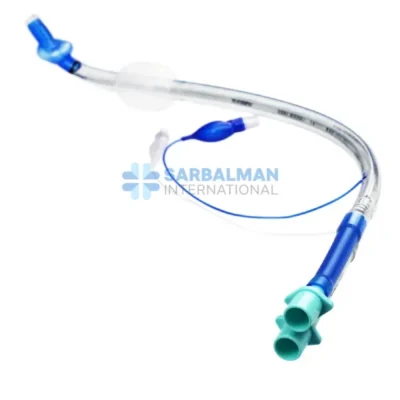
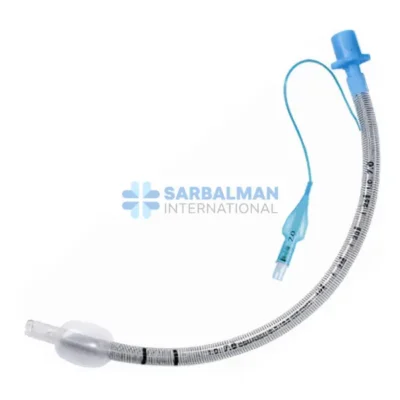
Succor Sub-Glottic Endotracheal tube
Free!
Description
The Succor Sub-Glottic Endotracheal Tube is an advanced airway management device designed for critically ill or mechanically ventilated patients, with the added benefit of sub-glottic secretion drainage. Positioned above the cuff and below the vocal cords, a dedicated suction port allows for continuous or intermittent removal of secretions from the sub-glottic space. This significantly reduces the risk of ventilator-associated pneumonia (VAP), a serious and common complication in intubated patients.
Made from medical-grade, transparent, and flexible PVC, this endotracheal tube combines the standard features of a cuffed tube with a dedicated lumen specifically engineered for secretion suctioning. It includes a soft, low-pressure cuff to ensure airway sealing, depth markers for precise placement, and a standard 15 mm connector to fit most ventilator circuits. The integrated suction line remains unobstructed, ensuring reliable and effective drainage without compromising ventilation.
Key Features & Benefits:
-
Sub-glottic suction port effectively removes accumulated secretions above the cuff to reduce VAP risk.
-
Soft, low-pressure cuff ensures a secure seal with minimal tracheal trauma.
-
Transparent construction allows visibility for secretion monitoring and placement verification.
-
Radiopaque line for accurate tube positioning using imaging.
-
Pilot balloon and one-way valve for easy cuff inflation and pressure monitoring.
-
Standard 15 mm universal connector ensures compatibility with all major respiratory systems.
Common Use Cases & Industry Applications:
-
Intensive Care Units (ICU) for prolonged mechanical ventilation.
-
High-risk patients for pulmonary infections.
-
Emergency airway management with infection prevention priority.
-
Post-operative recovery rooms for patients needing short-term ventilation support.
Comparative Advantage:
Unlike standard cuffed endotracheal tubes, the succor sub-glottic model offers continuous aspiration of potentially harmful secretions. This preventive function drastically reduces the incidence of VAP, improving patient outcomes and reducing ICU length of stay. It’s particularly valuable in long-term intubation scenarios and among immunocompromised patients.
Compliance & Quality Standards:
Typically produced in compliance with ISO 5361 and other international standards for airway devices, these tubes are generally latex-free, sterile, and manufactured using biocompatible materials to ensure safety and performance.
Functional Significance:
The Succor Sub-Glottic Endotracheal Tube is more than an airway device—it’s a proactive infection control solution. By enabling secretion removal before it enters the lungs, it elevates the standard of care in critical settings and aligns with modern ventilation strategies focused on both respiratory support and patient safety.